Moving Water Droplets: The Role of Atmospheric CO2 and Incident Radiant Energy in Charge Separation
Paper published December 6th, 2019
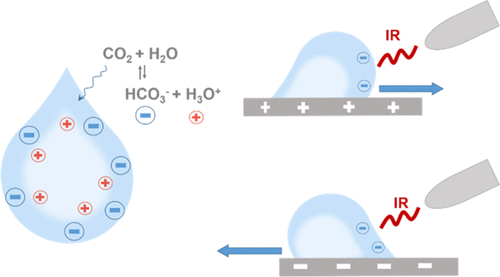
One of the characteristics of aqueous interfaces is their negative charge, whose origin is still a subject of scientific debate. In this work, we provide spectroscopic evidence that bicarbonate anions, from dissolution of atmospheric CO2, can be a source of negative charge at the air–water and/or solid–water interface. Also, interfacial charge separation, with a negatively charged droplet rim and positive charges gathering more toward the interior, makes water droplets receptive systems. We found that these droplets move in a controlled fashion because of electrostatic forces acting between droplets and a solid support possessing a static electric charge. A trigger used to induce droplets’ motion is IR emitted by different common objects. We interpret IR action as resulting from its ability to enhance the negative charge of the interfacial water. Droplets that leave negatively charged residues adsorbed to the solid, therefore acquiring a net positive charge, can defy the force of gravity and jump off the charged surface instead of falling, as observed in our experiments. Insights obtained from infrared emission of water agree with the possibility of excess protons residing in droplets after their contact with the solid. Our results show that (i) aqueous interfaces in contact with CO2 gas from the atmosphere (or possibly from cellular respiration inside of the organisms) acquire a negative surface charge, and (ii) infrared energy, abundant externally from the sun and internally from metabolic heat, can impact this process.